Q-17
REPORT: EXPERIMENT Q - VOLTAIC CELLS AND REDOX
REACTIONS
Name ____________________________________
Section ___________ Date ___________________
Laboratory Instructor________________________
1.
Assume that the Cd
2+
+ 2e
-
Cd half reaction has an electrode potential of -0.403 volts as
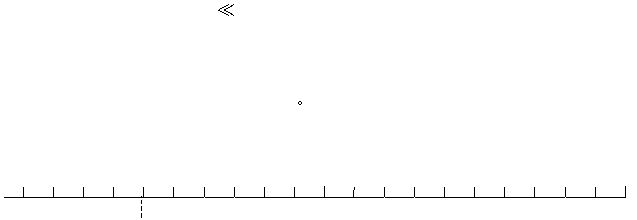
indicated on the line scale below (1 division = 0.1 volt). Use your results to place the
reduction potentials of the six half-cells that can be calculated on the scale shown below.
Show all calculations for the experimental E
red
potentials plotted.
0.0
Reduction Potentials
-0.8
+0.5
+1.0
Cd/Cd
2+
(-
0.403 V)