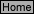Q-10
If a positive voltage is obtained, you have a spontaneous reaction or voltaic cell. This will allow
you to identify the anode and cathode for the voltaic cell. The electrode with the more positive
reduction potential (cathode) is connected to the positive lead of the voltmeter (red in color). If
you observe a negative potential for the cell, you have a nonspontaneous reaction or electrolytic
cell.
Record your data in the table provided on the Observations Sheet. Begin by connecting the Cu/Cu
2+
half-
cell with the Zn/Zn
2+
half-cell. To do this, firmly press the metal probe from the red lead to the Cu
electrode in the Cu/Cu
2+
half-cell and the metal probe from the black lead to the Zn of the Zn/Zn
2+
half-
cell.
NOTE: The metal probes MUST NOT touch the solution because they are plated with nickel and
may participate in an unwanted redox reaction.
When the leads are properly connected and the voltage is stable, record the voltage for this cell
combination on your Observations Sheet. Continue to connect the probe from the red lead to the copper
electrode and move the metal probe from the black lead on to the next half-cell. Record all of the values
on your Observations Sheet.
After you have compared the Cu/Cu
2+
half-cell to all of the other half-cells, move the red lead to the next
half-cell and compare it to all the other half-cells. Continue until you have tried all possible combinations,
recording all the voltages. The standard half-cell potentials for Ag, Cu, Pb, Cd, and Zn will be
determined from the data obtained. To check the results for each cell combination, make sure that the
absolute values of the voltaic and electrolytic cell potentials obtained are close. You may ask your
instructor to check your result.
C. Cell Voltages with C
(graphite)
/(I
-
, I2, I3
-
)
In the same 24-well plate used in Part B, prepare another half-cell using the standard iodine solution
(contains I
-
, I2, and I3
-
) from the stock bottle. Obtain a graphite electrode from your instructor and put it
into the well containing the standard iodine solution. Insert another strip of filter paper between this half-
cell and the KNO3 solution well to act as the salt bridge.