O-8
OBSERVATIONS SHEET - EXPERIMENT O
REDOX REACTIONS
PART I: Standardization Of Potassium Permanganate, KMnO
4
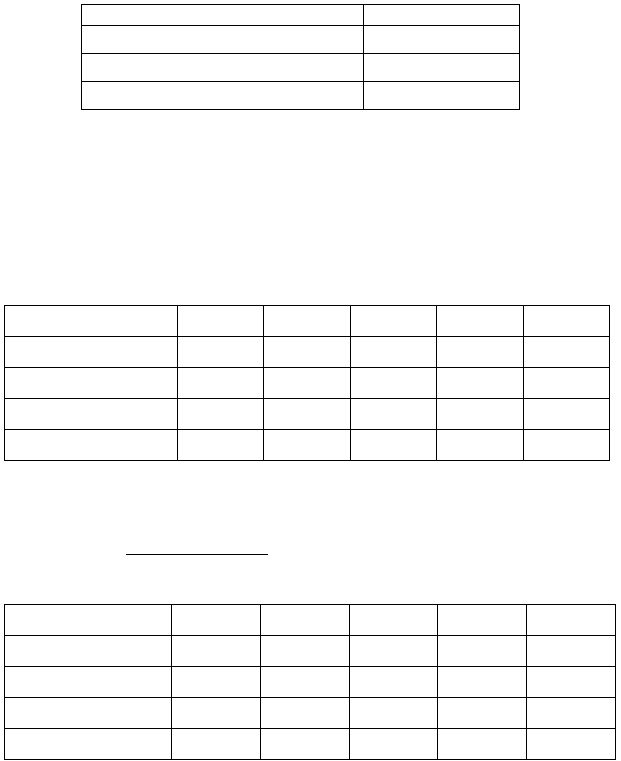
Mass (g)
mass of beaker + oxalic acid dihydrate
mass of beaker
mass of oxalic acid dihydrate
Calculation of the molarity of H2C2O
4
: (0.5 marks)
[H2C2O
4
] ___________M
Titration of oxalic acid with KMnO
4
: (buret readings to 0.02 mL)
Run 1
Run 2
Run 3
Run 4
(if necessary)
Run 5
(if necessary)
Final Volume (mL)
Initial Volume (mL)
Volume of KMnO
4
(mL)
color of end point
PART II: Titration of Unknown Hydrogen Peroxide Solution
Unknown Number:
Titration of hydrogen peroxide with KMnO
4
: (buret readings to 0.02 mL)
Run 1
Run 2
Run 3
Run 4
(if necessary)
Run 5
(if necessary)
Final Volume (mL)
Initial Volume (mL)
Volume of KMnO
4
(mL)
color of end point