O-2
Since the concentration of the KMnO
4
(titrant) used in this analysis must be accurately known, it must
be determined using a substance which concentration we know very accurately. This substance is
called a primary standard and the process is known as the “standardization” of the solution.
In this experiment, oxalic acid dihydrate, H2C2O
4
2H2O will be used as the primary standard to
standardize the potassium permanganate solution (titrant). Once the concentration of the KMnO
4
is
accurately known, this will allow one to determine the concentration of a solution of hydrogen peroxide,
which is serving as the reductant in this experiment.
Oxalic acid is a weak diprotic acid (K
a1
= 5.4 x 10
-2
, K
a2
= 5.2 x 10
-5
). As we will be working in a
strongly acidic solution, the solution will contain almost exclusively oxalic acid in this titration, reaction
(3):
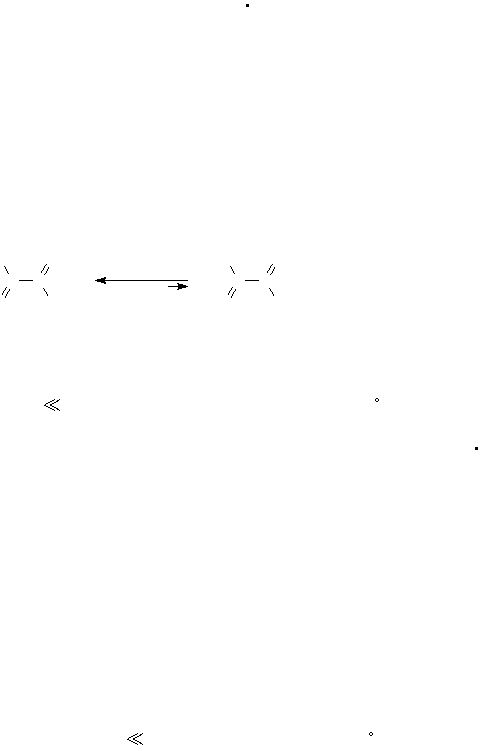
C
C
O
-
O
O
O
-
oxalate ion
C
C
O
HO
O
OH
+ 2 H
+
oxalic acid
(acidic solution)
(3)
It is oxalic acid which will be oxidized in this titration, reaction (4):
H2C2O
4(aq)
2 CO
2(g)
+ 2 H
+
(aq)
+ 2 e
-
-E
= +0.490 V
(4)
Oxalic acid can be obtained in a highly purified crystalline form as the dihydrate, H2C2O
4
2 H2O. As
well, oxalic acid is very stable both in air and when dissolved in water. In this experiment, oxalic acid
will be used as a primary standard. This means that when we dissolve 0.1000 moles of this substance in
1.000 L of water, the oxalic acid concentration will be 0.1000 M. Owing to their stability, primary
standards do not have to be standardized.
Hydrogen peroxide normally acts as an oxidizing agent (5). However, in this experiment, hydrogen
peroxide will act as a reducing agent owing to the presence of the strongly oxidizing MnO
4
-
ion.
Therefore reaction (6) gives the oxidation reaction that H2O2 will undergo:
H2O2
(aq)
+ 2 H
+
(aq)
+ 2 e
-
2 H2O
(l)
E
= +1.776 V
(5)