
A carving of Ashur-It, an early Assyrian city god (of Ashur)
dating from the Bronze Age.

A carving of Ashur-It, an early Assyrian city god (of Ashur)
dating from the Bronze Age.
practice exams | syllabus/outline | about chemed | contact Dusan
(c)
2003 Dusan Ristic-Petrovic
anything on chemed.ca is free for any non-commercial educational or humanitarian
purpose - please reproduce this notice
Felix
qui potuit rerum cognoscere causus
tetrammine
copper(II) ion, [Cu(NH3)4]2+
 |
 |
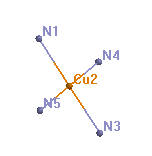 |
| space filling model of tetrammine copper(II) ion (surfaces show the approximate van der Waals radius of the atoms) | ball and stick model, showing square planar geometry at copper | model showing Cu and N atom labels |
This
week the molecule of the week is actually an ion, with a 2+ charge. However
the bonds within the ion are largely covalent. Electrons are shared
between the copper and the nitrogen atoms. The 'ammine' part is formed from
ammonia molecules, which act as ligands for the metal ion. Nitrogen, in
free ammonia, has a lone pair of electrons which can interact with empty
orbitals on the copper. Once formed, the resulting filled orbitals are shared
between copper and its ammine ligands.
This ion is referred to as a coordination complex, with the ammine ligands occupying four coordination sites on copper. Like ammonia, water has a lone pair (two pairs, actually) that it can donate to metal ions to form coordinate covalent bonds. This ability to donate electrons makes water or ammonia or other donor ligands Lewis bases; metal ions are Lewis acids, because they accept electrons.
Tetrammine copper (II) is formed when another complex, the blue-green aquo complex (which is what forms when copper (II) sulfate dissolves in water) is attacked by molecules of ammonia. Ammonia binds more strongly to copper than water (that is, it is a better donor of electrons) so it displaces the water molecules to give the deep blue [Cu(NH3)4]2+ ion. In solution there are also two molecules of water associated with [Cu(NH3)4]2+, but only loosely.
Coordination complexes of transition metals are essential components of many biomolecules; without them life on Earth could not exist. Chlorophyll contains a green magnesium complex, in which the magnesium ion is surrounded by nitrogen atoms. Hemoglobin, the oxygen carrier in the blood of most creatures, contains iron with nitrogen ligands. An oxygen molecule is held just strongly enough to the heme iron to be carried around the organism and released where needed. Carbon monoxide is a poison because it binds more tightly than oxygen, preventing its transport to the tissues.
Metal complexes are known for many simple organic molecules, both those with and without lone pairs. Even methane, which is an exceedingly weak Lewis base, can act as a ligand under certain circumstances.
More information...
coordination
complexes explained
chlorophyll
and photosynthesis
molecules
of life
the copper page
ancient
copper mines
